Key Product Detail – GemPureTM Select Water for Injection (WFI) Quality Water
GemPure™ Select Water for Injection (WFI) Quality Water (USP Grade)

GemPure™ Select Water for Injection (WFI) Quality Water (USP Grade)
GemPure™ Select Water for Injection (WFI) Quality Water (USP Grade) enables our customers to optimize their workflow and productivity – from development to commercial production – by providing WFI quality water that meets the USP monograph for Water for Injection packaged in bulk for commercial use.

WFI quality water that meets the needs of upstream and downstream bioprocessing and manufacturing.
Overview
For cell culture and other applications across the biopharma and advanced therapies workflows, GeminiBio offers GemPureTM Select Water for Injection (WFI) Quality Water. GemPureTM Select meets the USP monograph for Water for Injection packaged in bulk for commercial use. Available in 1-liter, 10-liter, 20-liter, 100-liter, and 200-liter package sizes. Custom manufacturing options are available.
Our GemPureTM Select Water Portfolio


GemPure™ Select Water for Injection (WFI) Quality Water (USP Grade) – 20 L BPC – Item: 901-001-021
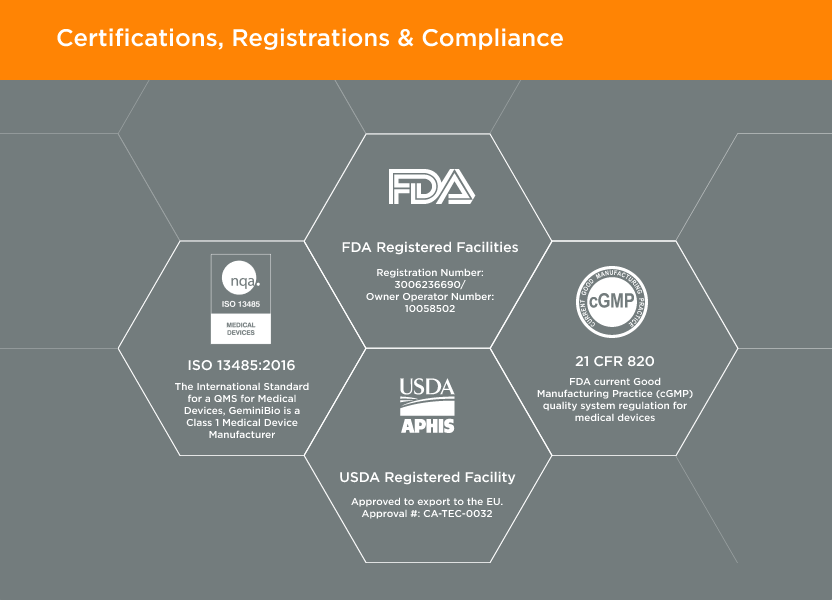
Our Integrated Quality Approach
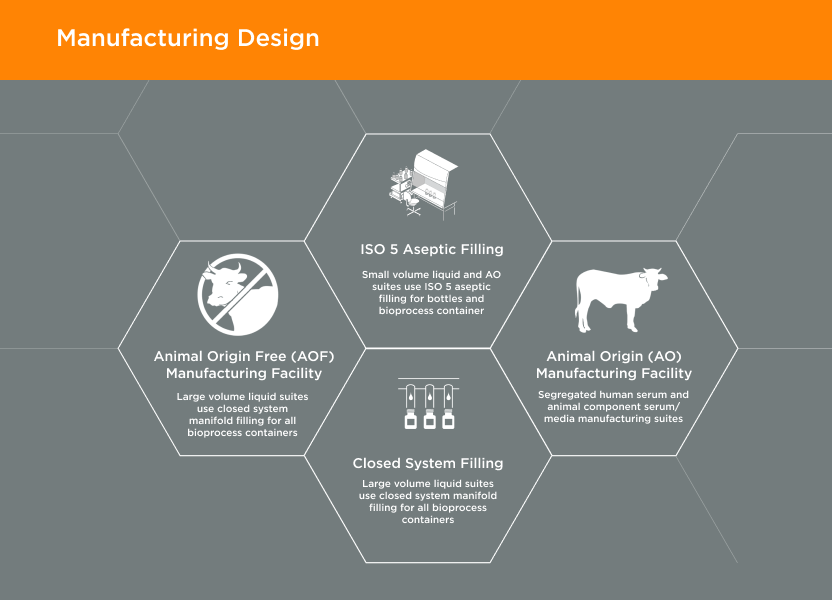
Manufacturing Design02
GeminiBio’s facilities are designed with current Good Manufacturing Practices (cGMP) and our customer’s quality requirements in mind. Our animal origin free (AOF) manufacturing facility is 1/4 mile away from our animal origin (AO) manufacturing facility. In addition, our cGMP warehouse is designed with flows for both personnel and materials, including segregation of AO and AOF materials.

How Can We Help?
Select from drop down
Contact: United States Sales Team
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America