Upstream and Downstream Liquids to Improve Your Workflow
Overview
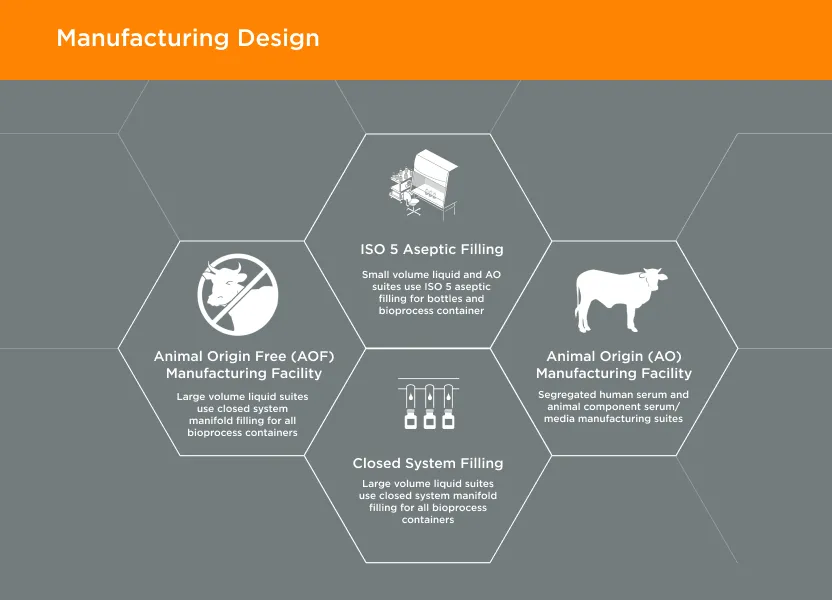
GeminiBio offers commonly used solutions, such as water for injection (WFI) quality water, water for irrigation (WFIr) quality water, and cell and molecular grade water “off the shelf” and in various package sizes to streamline customer workflows. For customers requiring custom solutions, GeminiBio offers custom manufactured cGMP process liquids and buffers in batch sizes from 10-liters to 10,000-liters.