Other Serum Options
Overview
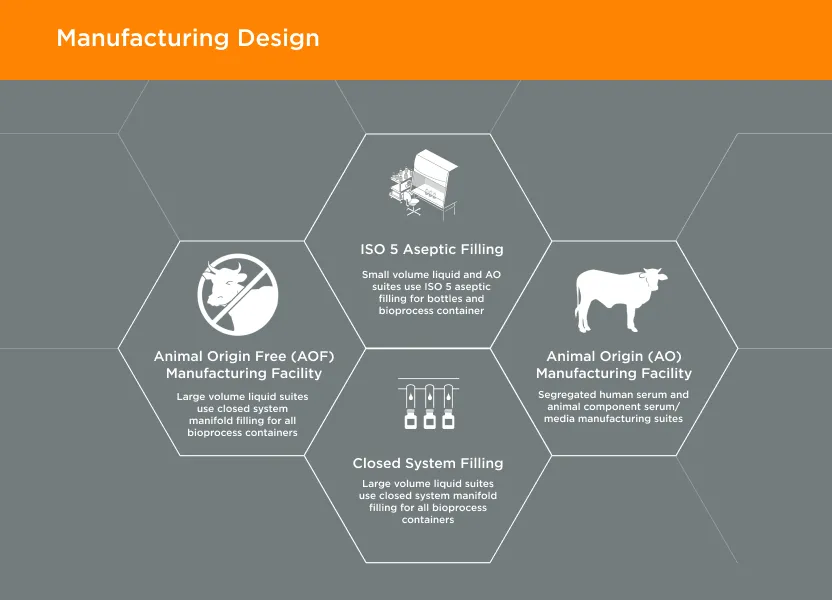
GeminiBio offers a wide range of other serums, including newborn calf, bovine calf, adult bovine, donor horse serum, and many other options for established cell lines. All GeminiBio serum products are manufactured under cGMP and include complimentary samples, lot reservation, heat inactivation, and cold storage to improve our customer’s workflow productivity, reproducibility, and speed.