To achieve our quality objectives, GeminiBio has developed and implemented a three tier Integrated Quality Approach that includes: Certifications, Registrations & Compliance, Manufacturing Design, and Risk Management. Our quality system and processes are applied consistently across our product portfolio – spanning products used in research applications to products used in the manufacturing process for commercial biologic therapies.
INTEGRATED QUALITY
Whether for research, development, or commercial production, our products are designed and manufactured to meet the stringent quality requirements of our customers.
Our Integrated Quality Approach


GeminiBio is committed to manufacturing products that consistently meet or exceed all industry and regulatory requirements or expectations.
Overview

GeminiBio is certified to ISO 13485:2016. As part of this certification, we are audited annually by the accrediting body to ensure that we maintain compliance. GeminiBio maintains an FDA registration under Class I Medical Devices with a registration number of 3006236690. GeminiBio is also aligned with 21 CFR Part 820: Quality System Regulation which helps ensures that we support our customer’s needs for current Good Manufacturing Practices (cGMP) compliance.
GeminiBio understands the needs of our customers when it comes to regulatory and compliance regarding the use of GeminiBio products. We work with our customers to obtain documentation required for filing with the FDA, EMA, and other various regulatory bodies throughout the world.

Personnel Flows
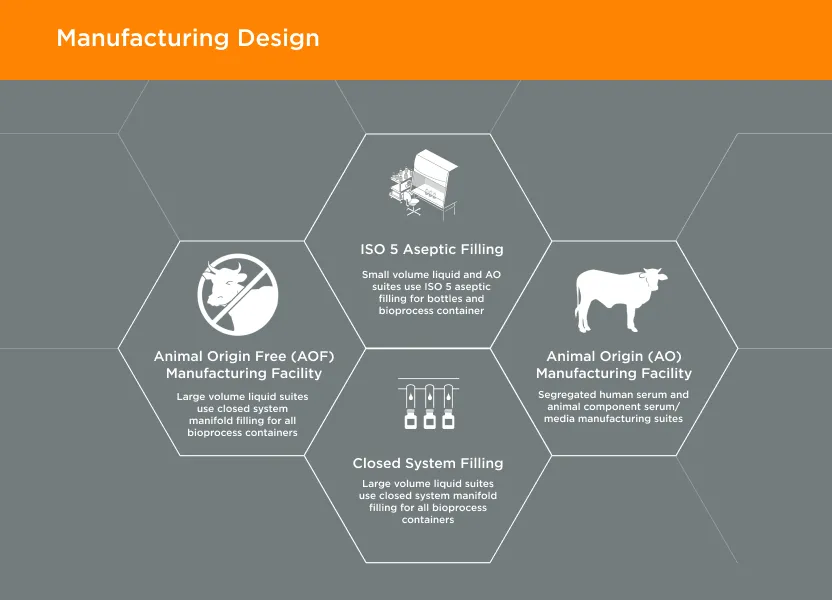
GeminiBio’s facilities are designed with current Good Manufacturing Practices (cGMP) in mind. The facilities are designed with flows for both personnel and materials, including segregation of animal origin (AO) and animal origin free (AOF) materials.
Personnel flows have been taken into consideration when designing the facility to mitigate potential cross contamination. Personnel go through increased levels of gowning requirements as they go from lower levels of cleanliness (clean non classified) to higher classifications (ISO 5) cleanroom environments. Personnel do not enter nor exit the facilities in the same areas as the product and waste streams and personnel are also qualified in gowning to work in the ISO7/ ISO 5 environments.
Material Flows
Material flows were also taken into consideration when designing the production facilities. Materials enter the facilities through a designated entry point, and they leave through a designated exit. There are designated areas for raw material storage prior to entering the production areas and all material containers are cleaned as they enter different classification areas. AO and AOF materials are segregated from the time they enter shipping and receiving until the time when finished product of AO or AOF classifications are shipped to customers. Each type of material is stored in designated areas and controlled based on material flows as to reduce the potential of cross contamination between the two types of materials and products. Production is performed in different facilities located approximately 1/4 mile from each other and AO materials do not enter the AOF facility. Raw material flow from shipping and receiving are also separated and do not cross paths to further minimize the potential of cross contamination.
Quality Control
Raw materials are received into the building after receiving activities, where critical materials are placed in QC Hold. While in QC Hold, Quality Control has responsibility for final disposition of raw materials after review of paperwork any required testing is confirmed to have been performed and meets the raw material specifications required. As part of the receiving process, raw materials are checked for compliance based on the receiving specifications. These specifications will list compliance requirements needed for the material based on the quality level (USP, EP, multi-compendial). Once Released by Quality Control, raw materials are removed for use under a first-in-first expiry (FIFE) system. Raw materials are documented in batch records with catalog and lot numbers to ensure traceability with the products that GeminiBio produces.
Quality Control is involved in the manufacturing process as an oversight group. The Quality Control department releases a production suite for manufacturing when the environment is confirmed to meet validated norms. Quality Control monitors the environment and personnel during the production process and confirms the environment did not change during the production process. Any in-process testing that cannot be performed by the production staff is performed by the Quality Control department. The manufacturing batch records are set up with Quality input (Quality by Design).


Supplier Management
GeminiBio understands the importance of our suppliers as we manufacture products for our end-customers. We have a rigorous Supplier Management program to approve suppliers based on their criticality impacting the manufactured final product. For high-risk suppliers, successful audits are required to receive full qualification as an Approved Supplier, and Quality Agreements or Change Notification Agreements are required to ensure we are able to secure transparency in our supply chain. Additionally, these audits help ensure that the goods and services that are obtained meet the requirements needed by our customers when our products are used within their workflows.
Change Management
GeminiBio has internal procedures to manage Change Controls. All operational changes within GeminiBio are governed by Change Management. The Change Management process considers all stakeholders impacted by the change, and the considerations needed to be addressed for the change. This can include Change Notification Agreements or Quality Agreements with customers or suppliers, or potentially customers that have purchased the product or service in recent history.
The Change Control process ensures that proper implementation is documented and controlled through the use of pre-implementation and post-implementation objective evidence, as well as control mechanisms that prevent the change from occurring without final approval.
Audits
Internal Audits
GeminiBio has an Internal Audit program, that is governed under ISO 13485:2016. This program reviews all required sections against the procedures performed under various departments and is performed annually. GeminiBio’s internal auditors are certified and trained to ISO 13485:2016 as lead auditors and are trained to ISO 14971 and ISO 9001.
Customer Audits
GeminiBio welcomes all customer audits and can support on-site audits throughout the year.
In addition to audits, GeminiBio maintains a supplier monitoring program. This includes periodic reviews of our suppliers to evaluate the recent performance in areas such as: On-Time Delivery, supplier Corrective Action Reports, and any changes to the business or relationships.



Select a Contact Option Bellow:
920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America

920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America

920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America

920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America

920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America

920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America

