Key Product Detail 100-912- GemCell Plus Xeno Free HSAB
GemCellTM Plus Xeno-Free HSAB

GemCellTM Plus Xeno-Free HSAB
GemCellTM Plus Xeno-Free Human Serum AB enables advanced therapy researchers and developers to streamline their workflow, allowing them to bring their end products to market quickly by providing human serum that is scalable, free of bovine components, uses therapeutic-grade human thrombin, and is rigorously tested.

For the cell culture of CAR-T and other cells where stringent regulatory requirements must be met, GeminiBio offers GemCellTM Plus Xeno-Free human AB serum. GemCellTM Plus Xeno-Free Human Serum is sourced plasma collected from healthy male donors of the AB serotype at FDA-licensed facilities in the United States.
Human AB serum that meets the needs of cell therapy researchers and developers.
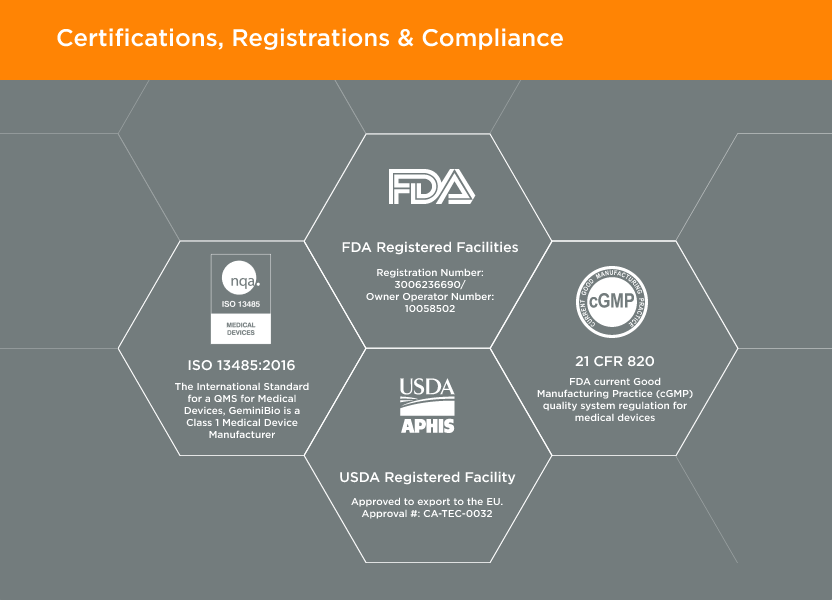
Our Integrated Quality Approach
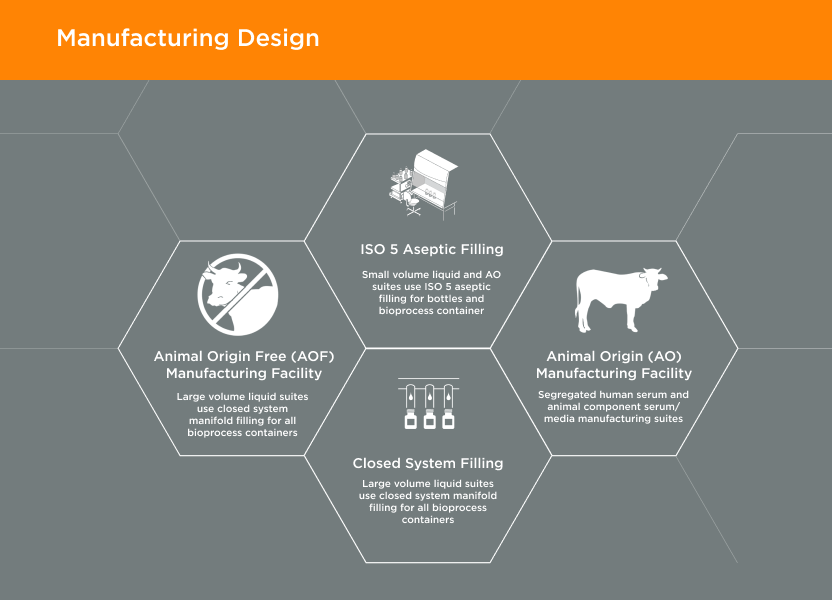
Manufacturing Design02
GeminiBio’s facilities are designed with current Good Manufacturing Practices (cGMP) and our customer’s quality requirements in mind. Our animal origin free (AOF) manufacturing facility is 1/4 mile away from our animal origin (AO) manufacturing facility. In addition, our cGMP warehouse is designed with flows for both personnel and materials, including segregation of AO and AOF materials.

How Can We Help?
Select from drop down
Contact: United States Sales Team
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America
920 Stillwater Rd Suite 130,
West Sacramento, CA 95605,
United States of America